
One way that chemists define atomic size is by using the atomic radius. Atomic size is defined in several different ways and these different definitions often produce some variations in the measurement of atomic sizes.īecause it is so difficult to measure atomic size from the nucleus to the outermost edge of the electron cloud, chemists use other approaches to get consistent measurements of atomic sizes. The region in space occupied by the electron cloud of an atom is often thought of as a probability distribution of the electrons and therefore, there is no well-defined "outer edge" of the electron cloud.
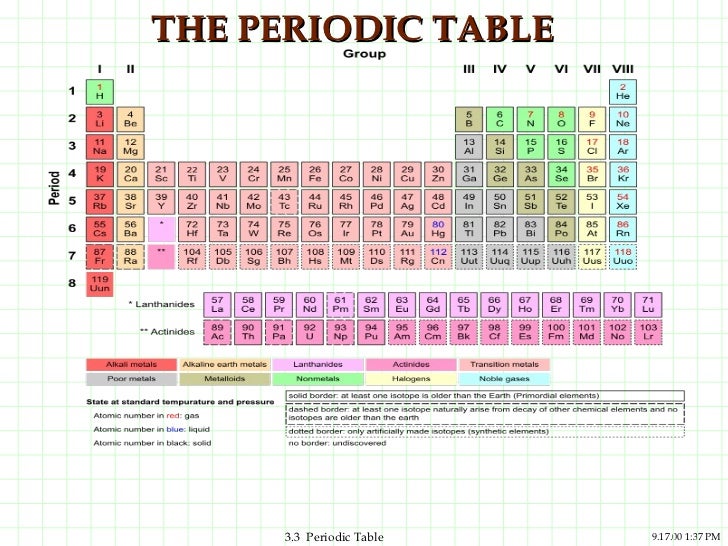

In the Periodic Table, there are a number of physical properties that are not really "similar" as it was previously defined, but are more trend-like. 5 For the Transition Elements, the Trend is Less Systematic.

4 Atomic Size in a Period Decreases from Left to Right.3 Atomic Size in a Column Increases from Top to Bottom.


 0 kommentar(er)
0 kommentar(er)
